Mpox
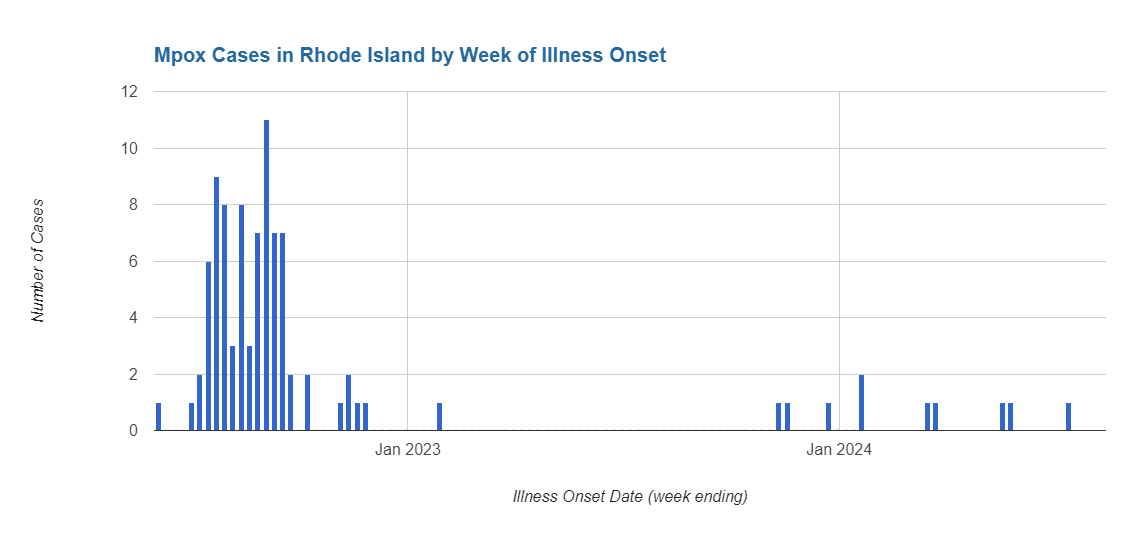
Mpox (Monkeypox Virus) is a rare, but potentially serious, viral illness which belongs to the orthopoxvirus family. Infection typically begins with flu-like symptoms and swelling of the lymph nodes and progresses to a rash on the face and body. Additional demographic data on Rhode Island's mpox cases is available on RIDOH's mpox data page.
Symptoms
Symptoms of mpox can include:
- Fever
- Headache
- Muscle aches and backache
- Swollen lymph nodes
- Chills
- Exhaustion
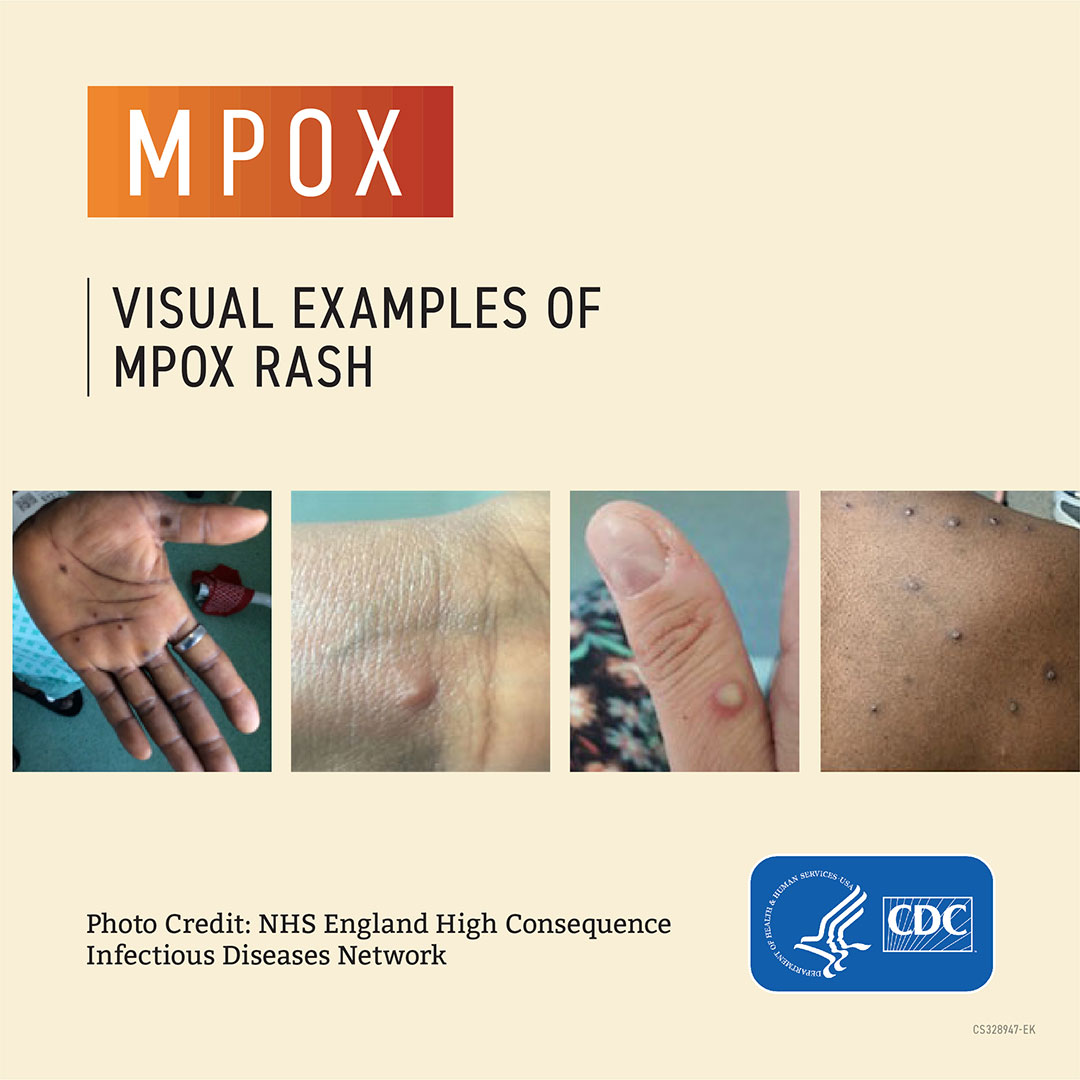
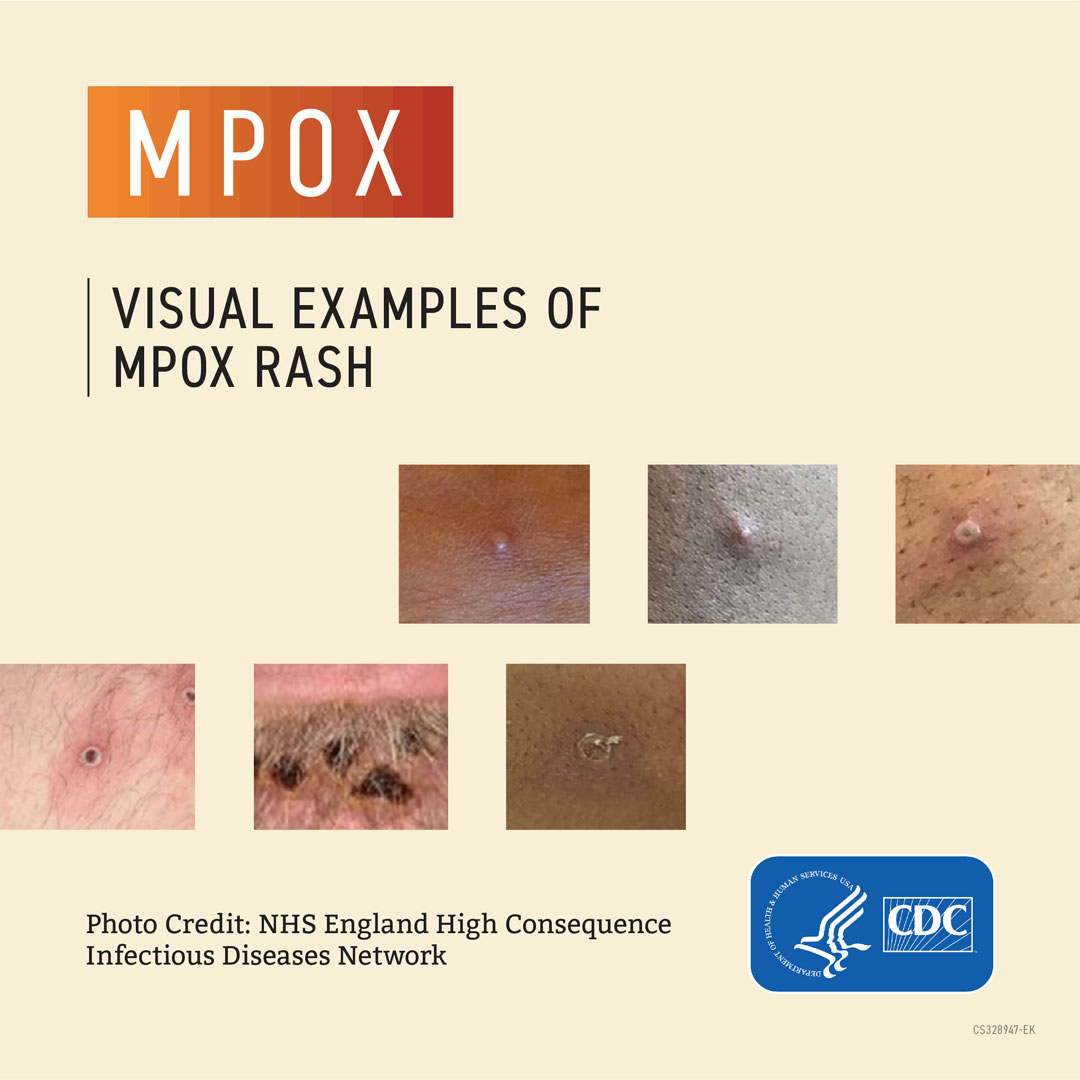
- A rash that can look like pimples or blisters that appears on the face, inside the mouth, and on other parts of the body, like the hands, feet, chest, genitals, or anus
Sometimes people get a rash first, followed by other symptoms. Others only experience a rash. The rash goes through different stages before healing completely. Most infections last two to four weeks and resolve on their own, but some cases can become severe.
Key points to know:
- Be aware of mpox and how it is transmitted;
- Recognize the signs and symptoms;
- Be careful if you are having multiple sexual partners;
- Get tested if you have any signs or symptoms.
Prevention
While many of the identified cases are within networks of self-identified gay and bisexual men, trans people, and men who have sex with men, people of any sexual orientation or gender identity can become infected and spread mpox.
Public awareness is important as the disease could spread within potentially larger groups or networks of people. RIDOH urges the media, government officials and the community at-large to avoid stigmatizing a particular group or person for mpox, but rather support those at highest risk and ensure that all communities remain vigilant.
Vaccination
Getting Vaccinated
The JYNNEOS mpox vaccine is now available on the commercial market. CVS and Walgreens now have the mpox vaccine in stock and vaccine scheduling is available on the CVS and Walgreens websites or speak to the pharmacy for other scheduling options. Speak to your health insurer and/or pharmacy to determine if there is any out-of-pocket cost. If you do not have insurance, one of the following clinics may be able to assist you with vaccination options available through the State Supplied Vaccine program:
- Open Door Health (Providence) – 401-648-4700
- Corliss Clinic at The Miriam Hospital - (Providence) – 401-793-2928 (At this location, vaccinations are available to eligible patients as part of a wellness appointment in the clinic. When calling, ask to schedule an appointment in the clinic. If you are specifically interested in the mpox vaccine, please indicate that so the provider will be aware. You still need to be evaluated by a provider. You will also be offered STI testing. The clinic has financial assistance services for patients who are uninsured or who have insurance with copays.)
Please remember that JYNNEOS is a two-dose series. Your second dose should be at least 28 days after your first dose.
Getting vaccinated before you are exposed to mpox is the best way to prevent disease. For best protection, two doses of JYNNEOS vaccine spaced 28 days apart are recommended. A CDC analysis indicated that among JYNNEOS vaccine-eligible men aged 18–49 years in 43 U.S. jurisdictions, mpox incidence among unvaccinated persons was 9.6 times as high as that among persons who had received 2 vaccine doses and 7.4 times as high as that among persons who had received only the first dose. Preliminary evidence indicates no difference in protection between subcutaneous and intradermal administration routes.
If you have already been exposed, getting vaccinated as soon as possible after exposure to someone with mpox (ideally within 4 days) may help prevent the disease, or make it less severe.
Please ask about administration fees before your vaccination. If you have insurance through someone else’s policy and are concerned about an Explanation of Benefits being sent to the policyholder, you may also contact your health insurer to find out the process under the law for requesting an explanation of benefits sent directly to you instead of the policy holder.
Eligibility
Anyone of any sexual orientation or gender identity who is at risk for mpox can get vaccinated. See below for details, including recommendations on who should get vaccinated. People who can be vaccinated include:
- People of any sexual orientation or gender identity who have or may have multiple or anonymous sex partners, or participate or may participate in group sex;
- People of any sexual orientation or gender identity whose sex partners are eligible per the criteria above;
- People who know or suspect they have been exposed to mpox in the last 14 days;
- Anyone else who considers themselves to be at risk for mpox through sex or other intimate contact;
- Healthcare workers who are caring for individuals with confirmed or suspected mpox or are testing or vaccinating people who are at risk for mpox.
- Laboratory workers who handle mpox specimens.
The current mpox outbreak has affected some populations more than others. In addition, the risk of getting mpox is higher among people who engage in certain activities or have certain health conditions. Based on this, the JYNNEOS vaccine is recommended for people who meet the above eligibility criteria and:
- Are men (cisgender or transgender) whose sex partners are men (cisgender or transgender), or gender diverse individuals; especially those who have been diagnosed with a sexually transmitted infection (STI) in the past 6 months;
- Are gender diverse, especially those who have been diagnosed with an STI in the past 6 months;
- Are cisgender women whose sex partners are men who have sex with men or who are gender diverse;
- Participate or may participate in sex parties or other events where there is minimal clothing and direct, frequent, or prolonged skin-to-skin contact;
- Are taking HIV pre-exposure prophylaxis (PrEP);
- Are living with HIV;
- Sex workers or others engaging in transactional sex (including sex in exchange for money, food, shelter or other goods) of any sexual orientation or gender identity.
About JYNNEOS
JYNNEOS is an FDA-approved vaccine for the prevention of smallpox and monkeypox in people age 18 or older who are considered at high-risk for smallpox or mpox infection.
JYNNEOS is a two-dose vaccine series. The second dose should be given at least 28 days after the first dose. Full protection is achieved 14 days after the second dose. You are fully protected 14 days after the second dose.
This vaccine does not contain the viruses that cause smallpox or mpox. The vaccine is made using the vaccinia virus, a virus is similar to but less harmful than the viruses that cause smallpox and mpox.
Treatment
Mpox and smallpox viruses are genetically similar, which means that antiviral drugs developed for smallpox may be used to treat mpox infections. Antivirals, such as tecovirimat (TPOXX), may be recommended for some people who are more likely to get severely ill, like patients with weakened immune systems. Not all patients are clinically eligible. In close consultation with patients’ healthcare providers and the CDC, RIDOH is authorizing and coordinating the use of treatment for eligible patients.
CDC Information
For Healthcare Professionals
Healthcare professionals should review RIDOH’s latest provider advisories related to mpox and CDC's information for healthcare professionals.